Ebola virus (EBOV)
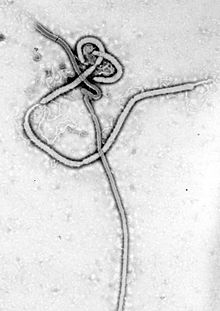 | |||
| Ebola virus electron micrograph |
| Virus classification | |||||
|---|---|---|---|---|---|
| Group: | Group V ((-)ssRNA) | ||||
| Order: | Mononegavirales | ||||
| Family: | Filoviridae | ||||
| Genus: | Ebolavirus | ||||
| Species: | Zaire ebolavirus | ||||
Ebola virus (EBOV) causes severe disease in humans and in nonhuman primates in the form of viral hemorrhagic fever. EBOV is a select agent, World Health Organization Risk Group 4 Pathogen (requiring Biosafety Level 4-equivalent containment), National Institutes of Health/National Institute of Allergy and Infectious Diseases Category A Priority Pathogen, Centers for Disease Control and Prevention Category A Bioterrorism Agent, and listed as a Biological Agent for Export Control by the Australia Group.
| read up more... in any case you are traveling to the following countries... :) Prevention they say, better than cure! |
Ebola virus (abbreviated EBOV) was first described in 1976. Today, the virus is the single member of the species Zaire ebolavirus, which is included into the genus Ebolavirus, family Filoviridae, order Mononegavirales. The name Ebola virus is derived from the Ebola River (a river that was at first thought to be in close proximity to the area in Zaire where the first recorded Ebola virus disease outbreak occurred) and the taxonomic suffix virus. Ebola virus is pronounced ɛ’bɒlə vɑɪrəs (IPA) or eh-bo-luh v-eye-ruhs in English phonetic notation. According to the rules for taxon naming established by the International Committee on Taxonomy of Viruses (ICTV), the name Ebola virus is always to be capitalized, but is never italicized, and may be abbreviated (with EBOV being the official abbreviation).
Previous designations
Ebola virus was first introduced as a possible new "strain" of Marburg virus in 1977 by two different research teams. At the same time, a third team introduced the name Ebola virus. In 2000, the virus name was changed to Zaire Ebola virus, and in 2005 to Zaire ebolavirus. However, most scientific articles continued to refer to Ebola virus or used the terms Ebola virus and Zaire ebolavirus in parallel. Consequently, in 2010, the name Ebola virus was reinstated. Previous abbreviations for the virus were EBOV-Z (for Ebola virus Zaire) and most recently ZEBOV (for Zaire Ebola virus or Zaire ebolavirus). In 2010, EBOV was reinstated as the abbreviation for the virus.Virus inclusion criteria
A virus of the species Zaire ebolavirus is an Ebola virus if it has the properties of Zaire ebolaviruses and if its genome diverges from that of the prototype Zaire ebolavirus, Ebola virus variant Mayinga (EBOV/May), by ≤10% at the nucleotide level.Disease
Virology
Structure
Electron micrographs of EBOV show them to have the characteristic threadlike structure of a filovirus. BOV VP30 is around 288 amino acids long. The virions are tubular in general form but variable in overall shape and may appear as the classic shepherd's crook or eyebolt, as a U or a 6, or coiled, circular, or branched; laboratory techniques, such as centrifugation, may be the origin of some of these formations.Virions are generally 80 in diameter with a lipid bilayer anchoring the glycoprotein which projects 7 to 10 nm long spikes from its surface. They are of variable length, typically around 800 nm, but may be up to 1000 nm long. In the center of the virion is a structure called nucleocapsid, which is formed by the helically wound viral genomic RNA complexed with the proteins NP, VP35, VP30, and L. It has a diameter of 80 nm and contains a central channel of 20–30 nm in diameter. Virally encoded glycoprotein (GP) spikes 10 nm long and 10 nm apart are present on the outer viral envelope of the virion, which is derived from the host cell membrane. Between envelope and nucleocapsid, in the so-called matrix space, the viral proteins VP40 and VP24 are located.Genome
Each virion contains one molecule of linear, single-stranded, negative-sense RNA, 18,959 to 18,961 nucleotides in length. The 3′ terminus is not polyadenylated and the 5′ end is not capped. It was found that 472 nucleotides from the 3' end and 731 nucleotides from the 5' end are sufficient for replication.It codes for seven structural proteins and one non-structural protein. The gene order is 3′ – leader – NP – VP35 – VP40 – GP/sGP – VP30 – VP24 – L – trailer – 5′; with the leader and trailer being non-transcribed regions, which carry important signals to control transcription, replication, and packaging of the viral genomes into new virions. The genomic material by itself is not infectious, because viral proteins, among them the RNA-dependent RNA polymerase, are necessary to transcribe the viral genome into mRNAs because it is a negative sense RNA virus, as well as for replication of the viral genome. Sections of the NP and the L genes from filoviruses have been identified as endogenous in the genomes of several groups of small mammals.Replication
Being acellular, viruses do not grow through cell division; instead, they use the machinery and metabolism of a host cell to produce multiple copies of themselves, and they assemble in the cell.- The virus attaches to host receptors through the glycoprotein (GP) surface peplomer and is endocytosed into macropinosomes in the host cell Viral membrane fuses with vesicle membrane, nucleocapsid is released into the cytoplasm
- Encapsidated, negative-sense genomic ssRNA is used as a template for the synthesis (3' – 5') of polyadenylated, monocistronic mRNAs
- Using the host cell's machinery, translation of the mRNA into viral proteins occurs
- Viral proteins are processed, glycoprotein precursor (GP0) is cleaved to GP1 and GP2, which are heavily glycosylated. These two molecules assemble, first into heterodimers, and then into trimers to give the surface peplomers. Secreted glycoprotein (sGP) precursor is cleaved to sGP and delta peptide, both of which are released from the cell.
- As viral protein levels rise, a switch occurs from translation to replication. Using the negative-sense genomic RNA as a template, a complementary +ssRNA is synthesized; this is then used as a template for the synthesis of new genomic (-)ssRNA, which is rapidly encapsidated.
- The newly formed nucleocapsids and envelope proteins associate at the host cell's plasma membrane; budding occurs, destroying the cell.
No comments:
Post a Comment